-
Antibody basics training
Part 1: Choosing the right antibody
Welcome to our training series on how to choose and use antibodies. Here we’ll guide you through topics such as selecting the right antibodies for your needs, handling and storing antibodies, antibody validation, and troubleshooting when things go wrong.In Part 1 of our series on antibody basics, we cover the main points of consideration when you first select an antibody, as this is crucial for specific and sensitive experiments. You’ll learn about antibody structure and isotypes, experimental setup options, primary antibodies, their direct and indirect detection methods, and secondary antibody selection.
Part 1 overview1.1 Antibody structure and isotypes
1.2 Choosing a suitable application
1.3 Choosing your primary antibody
1.4 Direct vs indirect detection methods
1.5 Choosing your secondary antibody
1.1 Antibody structure and isotypesAntibodies, also known as immunoglobulins (Ig), are large, Y-shaped glycoproteins produced by B-cells as a primary immune defense. Antibodies specifically bind unique molecules of a pathogen, called antigens.
Antibodies exist as one or more copies of a Y-shaped unit composed of four polypeptide chains (Figure 1). Each Y unit contains two identical copies of a heavy chain (H) and two identical copies of a light chain (L), which are different in their sequence and length. The top of the Y shape contains the variable domain (V), also known as the fragment antigen-binding (F(ab)) region. This region binds tightly and specifically to an epitope on a given antigen.
The base of the antibody consists of constant domains (C) and forms the fragment crystallizable region (Fc). This region is important for the function of the antibody during an immune response. In addition, dye and enzymes can be covalently linked to antibodies on the Fc portion of the antibody for experimental visualization.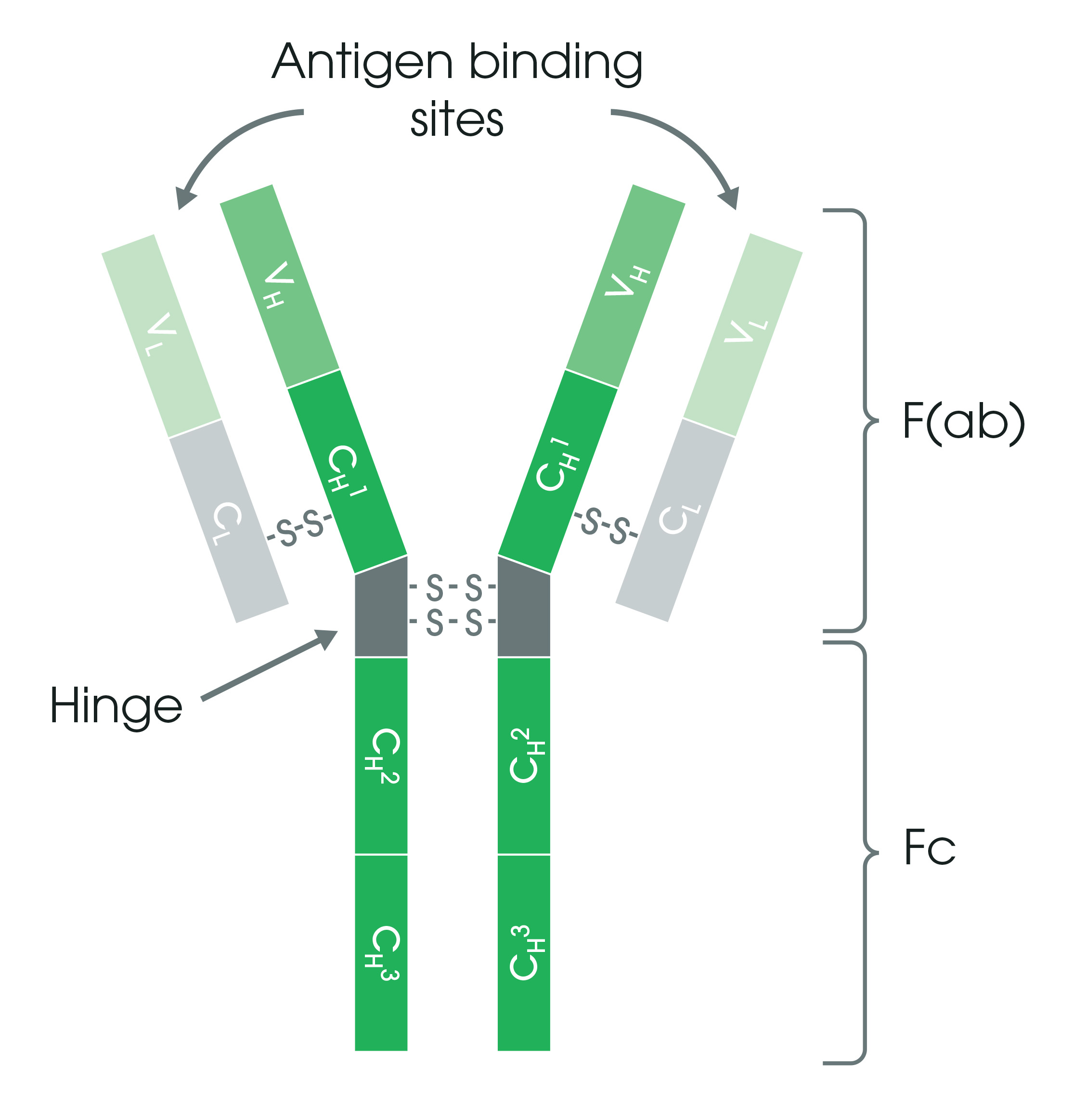
Figure 1. Antibody structure. The Y-shaped antibody is joined in the middle by a flexible hinge region. Antigen binding occurs at the variable domain (V) consisting of immunoglobulin heavy (H) and light chains (L). The base of the antibody consists of constant domains (C).
The light chains of an antibody can be classified as either kappa (κ) or lambda (λ) type based on small differences in the polypeptide sequence. The heavy chain type determines the overall class or isotype of each antibody.
In mammals, antibodies are divided into five isotypes: IgG, IgM, IgA, IgD, and IgE. Each isotype has a unique structure, as depicted in Figure 2. The isotypes vary based on the number of Y units and the type of heavy chain. They will also differ in their biological properties, functional locations, and ability to deal with different antigens.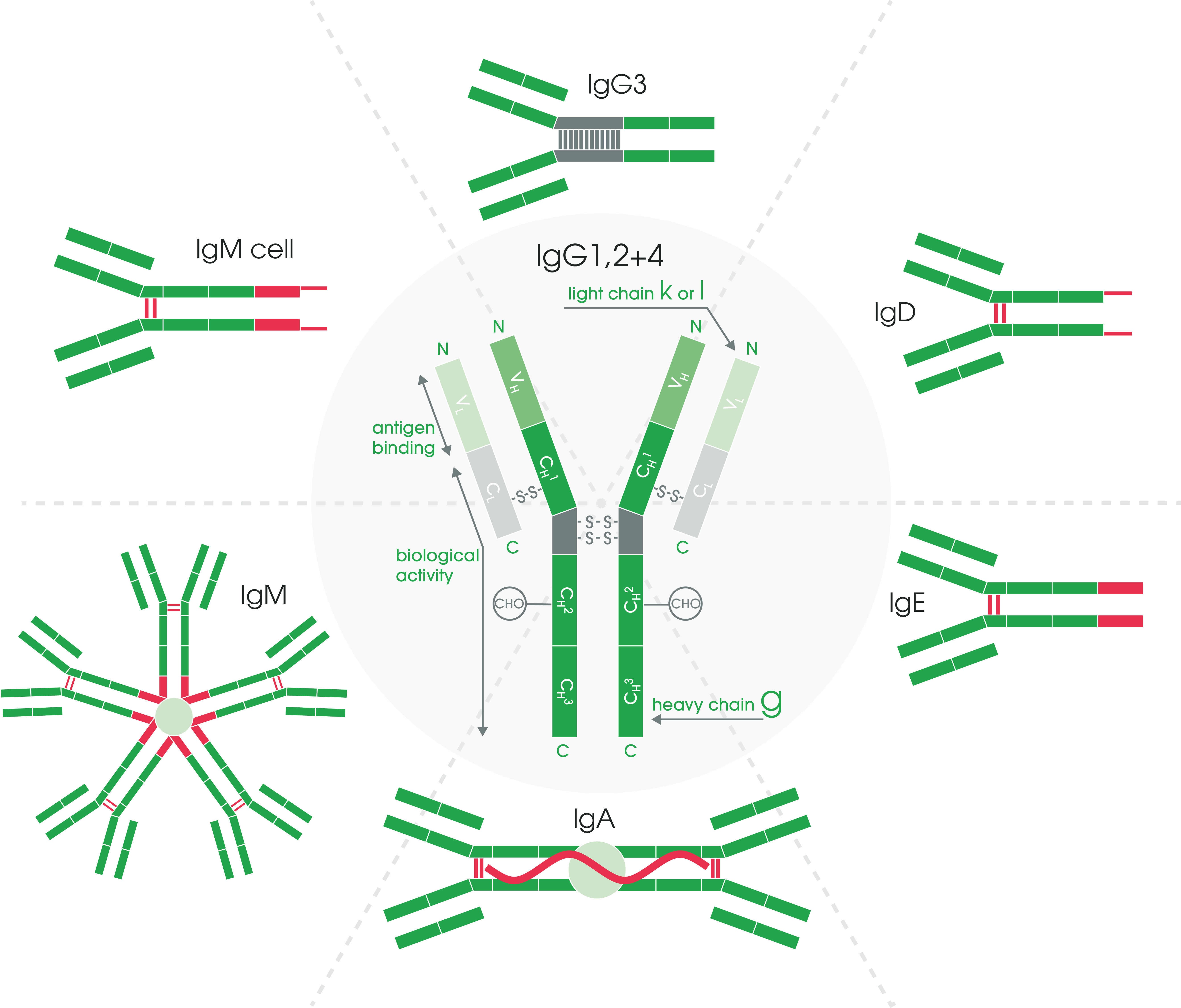
Figure 2. Antibody structure and isotypes.
1.2 Choosing a suitable applicationWhat are you studying? Consider using Use Sample Data type Localization IHC Determine cellular localization Fixed cells
and tissuesQual. and semi-quant. ICC Determine subcellular localization Fixed cells Qual. and semi-quant. Conc. WB Measure relative protein levels Cell and tissue lysates Semi-quant. ELISA Quantify proteins levels accurately Serum, plasma,
live or fixed
cells or tissueQual. and quant. Interaction ELISA Examine molecular interactions Serum, plasma, cells or tissue Qual. and quant. Expression FCM or FACS Analyze cell properties such as size and expression of surface markers Fixed or live cells Quant. Identification ELISPOT Identify specific cell(s) secreting analyte of interest Live cells Qual. and quant.
Conc. = concentration, IHC = immunohistochemistry, ICC = immunocytochemistry, ELISA = enzyme-linked immunosorbent assay, FACS = fluorescence-activated cell sorting, FCM= flow cytometry, ELISPOT = enzyme-linked immunospot, Qual. = qualitative, Quant. = quantitative, WB = western blot
1.3 Choosing a primary antibodyWhen choosing a primary antibody, consider the following:
Clonality
When selecting an antibody, one of the most important factors to consider is clonality. Clonality is determined by whether the antibodies come from different B-cells (polyclonal antibodies) or identical B-cells derived from a parent clone (monoclonal antibodies). These antibodies have distinct advantages and limitations.
Polyclonal antibodies are a heterogeneous mix of antibodies derived from the immune response of multiple B-cells, and each one recognizes a different epitope on the same antigen. Since polyclonal antibodies detect a range of epitopes on a protein, they are prone to a higher risk of batch-to-batch variability than monoclonal antibodies. However, due to their lack of specificity to one epitope, polyclonal antibodies are more tolerant of minor antigen changes than monoclonal antibodies.
Monoclonal antibodies come from a single B-cell parent clone and, therefore, only recognize a single epitope per antigen. Monoclonal antibodies have high specificity for their target, low non-specific cross-reactivity, and minimal batch-to-batch variations.
In this video, you can learn the difference between polyclonal and monoclonal antibodies, using the example of antibodies detecting histone modifications.
Monoclonal antibodies are typically made using B-cells from an immunized animal, which are fused to an immortalized cell line to form a hybridoma that secretes the desired antibody clone. This hybridoma technique produces highly consistent, specific, and sensitive monoclonal antibodies in large quantities. However, over time, hybridoma cell lines can experience genetic drift, resulting in slight variations to the antibodies produced.
There is also a growing demand for antibodies against difficult targets, ie toxins, nucleotides, and membrane-bound proteins, that can’t always be made with this in vivo model.
Recombinant antibodies are produced in vitro by cloning antibody genes for immune-specific heavy and light antibody chains into high-yield expression vectors. These vectors are then introduced into expression hosts (eg bacteria, yeast, or mammalian) to generate the recombinant monoclonal antibodies.
Recombinant antibodies offer several advantages over both traditional monoclonal and polyclonal antibodies:
• Improved consistency and reproducibility due to very low batch-to-batch variability
• Improved sensitivity and specificity achieved through antibody engineering
• Ease of scalability
• Animal-free high-throughput selection
To learn more about recombinant antibodies, their advantages, and production process, read here. Watch this video for a more in-depth review of three antibody formats and learn how the choice of antibody format may influence the consistency of your results.
Species
Ideally, the species of your sample should be different from the species the antibody was raised in. With applications like western blot that use a cell lysate without any endogenous immunoglobulin (IgG), or experiments that use primary conjugated antibodies, you don’t have to worry about this. If you have to work on the same species as your antibody (eg mouse-on-mouse), we can help you to modify your staining protocol.
A note on non-model organisms:
If you work in a non-model organism, you may need to use an antibody that hasn’t been tested in your species. In many cases, the protein is conserved enough across several species and, therefore, can be recognized with an antibody that was not validated in this species.
If there’s no alternative, we recommend checking the sequence alignment of the immunogen with the protein you’re interested in. You can find sequences on the Swiss-Prot protein database link on the online datasheet. Some websites provide tools for calculating the percentage alignment – check out the multiple sequence alignment tool CLUSTALW.
Take a copy of the immunogen sequence of the antibody and align it with your protein sequence from the species you would like to test. An alignment score of over 85% is a good indication that an antibody may cross-react. However, even with a score of over 85%, there’s no guarantee for how well the antibody will perform. You’ll need to run several controls to make sure it works as intended.
Immunogen considerations
Antibodies are generated by immunizing host animals with an immunogenic substance. Immunogens can be full-length proteins, protein fragments, peptides, whole organisms (eg bacteria), or cells. The immunogen is generally described on the datasheet.
The immunogen of the antibody will define which region of the protein antibody binds. Watch the short video below to learn why you should think about the immunogen before selecting an antibody.
Carrier-free antibody formats
Normally, antibodies are stored in a solution of phosphate-buffered saline (PBS) with several key carriers: bovine serum albumin (BSA), glycerol, and sodium azide. While BSA and azide are essential components for maintaining antibody stability and preventing antibody contamination, these components can also hinder effective conjugation to fluorochromes, metals, and enzymes.
BSA competes with the primary antibody to attach to the label of interest, which greatly reduces the conjugation efficiency. The presence of sodium azide in the antibody solution can be toxic to cells, limiting the antibody's use in cell culture. Therefore, if you intend to conjugate your primary antibody or use it for staining of live cells, we recommend you to choose antibodies in carrier-free formats, ie BSA-free and sodium azide-free.
A checklist for choosing primary antibodies
• Check antibody clonality, application suitability, host species, and species reactivity – you can find these on the supplier’s datasheet
• Take a look at the images and data provided by the supplier – do the data look robust?
• Read customer reviews and any recent papers the antibody is cited in – these should also be provided on the supplier datasheet and will give a good idea of how the antibody performs
1.4 Direct vs indirect detection methodsThe method of detecting a primary antibody that is bound to the antigen of interest can be either direct or indirect.
In direct detection methods, the primary antibody is directly conjugated to a label. During indirect detection, the primary antibody is bound by a labeled secondary antibody that has been raised against the host species of the primary antibody. Indirect methods may also include amplification steps to increase signal intensity. The choice of direct or indirect detection is often prescribed by the expression level of the target antigen.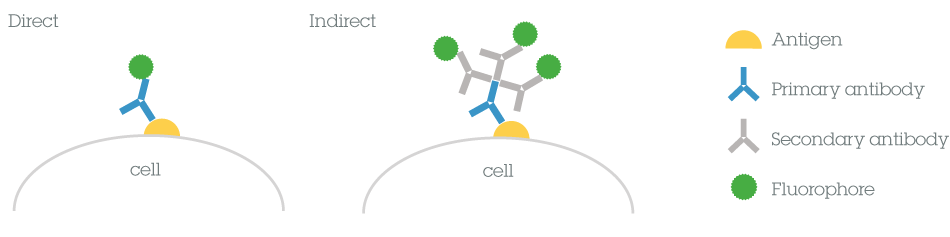
Direct vs indirect
Both methods have several benefits and limitations to consider before choosing the most appropriate method for your experiments.
Direct Indirect Time Usually shorter as it only requires one labeling step. Using a conjugated secondary antibody results in additional steps and a longer time. Complexity Fewer steps in the protocol makes this a simpler method. Added complexity results from having to select the appropriate secondary antibody (or combinations of antibodies in multiplex experiments). Sensitivity May seem weak when compared to indirect methods as there is no signal amplification provided by secondary antibodies. Several secondary antibodies may bind to the primary antibody resulting in an amplified signal. Background Non-specific binding is reduced. Samples with endogenous immunoglobulins may exhibit a high background.
Watch this webinar, if you want to learn more about the benefits and limitations of direct versus indirect methods in multiple applications, including cellular imaging and flow cytometry.
1.5 Choosing your secondary antibodyIf you are not using a directly conjugated primary antibody, then you will need to select an appropriate secondary antibody.
Secondary antibodies are raised against the species, isotype of the primary antibody and are used to detect the primary antibody by binding to it in more than one place. Here are some things that you need to consider when choosing a secondary antibody for your experiment.
Host species
The secondary antibody binds specifically to the IgG of the host species used to generate the primary antibody. For example, if you use a primary antibody raised in rabbit, you will need an anti-rabbit secondary antibody raised in a host species other than rabbit (eg donkey anti-rabbit secondary).
Class/subclass of antibody
The secondary antibody has to be directed against the isotype of the primary antibody. Polyclonal primary antibodies are typically raised in rabbit, goat, sheep or donkey and are generally IgG isotypes. Therefore, the secondary antibody will usually be an anti-IgG H&L (heavy & light chains) antibody.
Monoclonal primary antibodies are commonly raised in mouse, rabbit, and rat. For example, if the primary monoclonal antibody is a mouse IgG1, you will need an anti-mouse IgG or a less-specific F(ab) fragment anti-mouse IgG.
Conjugate selection for different applications
To visualize the presence of the target protein, secondary antibodies are conjugated to fluorescent dyes/proteins, enzymes, and biotin.
• Fluorescent labels emit light in the visual range when excited by light of a specific wavelength. There are several available, all with their own excitation and emission characteristics.
• Enzymatic labels, such as horseradish peroxidase (HRP) and alkaline phosphatase (AP), form a colored precipitate when combined with the appropriate substrate.
• Biotinylated antibodies are useful for amplification of signal when followed by an avidin-biotin-enzyme or fluorochrome complex (commonly abbreviated as ABC reagent), or avidin or streptavidin conjugated to an enzyme or fluorochrome.
The conjugate choice depends on the experimental application. For applications such as ELISA or western blotting, enzyme-linked secondary antibodies tend to be the most popular, whereas, for flow cytometry or immunofluorescence, there is a preference for secondary antibodies conjugated to fluorescent proteins or dyes such as Alexa Fluor® dyes.
Below are some suggested secondary antibodies for the main applications you’re likely to use.Secondary antibodies Enzyme Fluorochrome IHC HRP, HRP polymer, Biotin (avidin/ streptavidin conjugated to enzyme or fluorochrome) Alexa Fluor®, Cy® dyes, FITC, PE ICC - Alexa Fluor®, Cy® dyes, FITC, PE Western blot HRP, AP IRDye®, Alexa 680, Alexa 790 ELISA or ELISPOT HRP, Biotin (avidin/ streptavidin conjugated to enzyme or fluorochrome) - Flow cytometry or FACS - Alexa Fluor®, Cy® dyes, FITC, PE
IHC = immunohistochemistry, ICC = immunocytochemistry, FACS = fluorescence-activated cell sorting, HRP = horseradish peroxidase, AP = alkaline phosphatase
Pre-adsorbed secondary antibodies
Pre-adsorbed secondary antibodies are ideal for eliminating species reactivity in multi-color experiments when several primary antibodies and their corresponding secondary antibodies are used simultaneously. Pre-adsorption (also referred to as cross-adsorption) is an extra purification step introduced to increase the specificity of an antibody. The pre-adsorption process reduces the risk of cross-reactivity between the secondary antibody and endogenous immunoglobulins present on cell and tissue samples.
F(ab) fragment antibodies
F(ab) and (Fab')2 fragment antibodies eliminate non-specific binding between Fc portions of antibodies and Fc receptors on cells (such as macrophages, dendritic cells, neutrophils, NK cells, and B cells) and penetrate tissues more efficiently due to their smaller size.
Quick tips on choosing secondary antibodies• Select the brightest fluorochrome to label a protein with the lowest expression levels
• All secondary antibodies should come from the same host species when you use multiple labels
• If you use serum from the same host species of the secondary to block, this will significantly reduce background
• Pre-adsorbed secondary antibodies are useful for multicolor analysis to ensure low cross-species reactivity
• Fragment antibodies are smaller and penetrate tissues more efficiently (useful for IHC)
• Biotin conjugates can detect low-abundance proteins
For more information on how to choose the right secondary antibody for your experiment, get the full guide.Summary
And that's the end of Part 1. Now you should be fully prepared to choose the appropriate antibody for your next experiment.
In Part 2, you'll get all the information you need for storing and handling your antibody.
You’ll learn about:
• How to properly store and handle your antibodies, including freezing and aliquoting
• Specific storage conditions for conjugated antibodies
• Storage and handling FAQs
We’ll also answer the most important question of all: “Help! I left my antibody out on the bench overnight – will it still work?”
Start Part 2 now!