-
Fluorescent imaging training
Part 2: choosing your antibody
Welcome to our training series on fluorescent imaging. We’ll show you imaging basics and essential steps before moving on to optimization, troubleshooting, and more advanced techniques.Selecting the right antibody is crucial for specific and sensitive experiments. In Part 2 of our training series, you'll learn about different types of primary and secondary antibodies, different staining methods, and a guide to fluorophores, so you can make the best decision when it comes to your experiments.
Overview
2.1 Direct vs indirect immunofluorescence
2.2 Choosing a secondary antibody
2.3 Conjugate selection
2.4 Choosing your fluorophore2.1 Direct vs indirect immunofluorescence
Immunofluorescence (IF) makes use of antibodies to label a specific target antigen with a fluorescent dye (also called a fluorophore or fluorochrome). There are two main types of IF methods depending on whether the fluorophore is conjugated to the primary or the secondary antibody.
1) Direct IF uses a single antibody directed against the target of interest that is directly conjugated to a fluorophore
2) Indirect IF uses two antibodies: an unconjugated primary antibody directed against the target of interest, and a fluorophore-conjugated secondary antibody that binds the primary antibody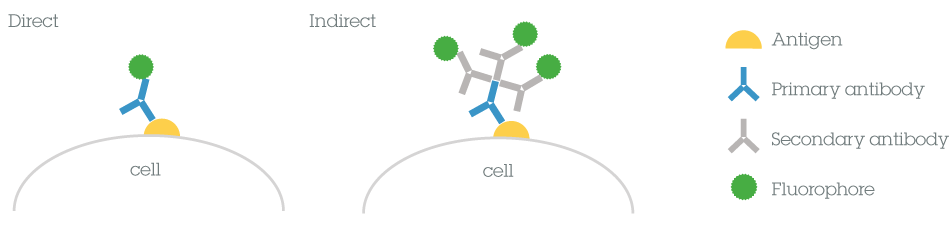
Each method has a number of benefits and limitations to consider before choosing the most appropriate method for your experiments.Direct vs indirect
Time Usually shorter as they only require one labeling step. Using a conjugated secondary antibody to detect the primary antibody results in additional steps and longer time. Complexity Fewer steps in the protocol create a simpler method for direct IF. The added complexity in indirect methods may result from having to select the appropriate secondary antibody (important in multiplex experiments where several secondary antibodies are needed). Sensitivity May seem weak when compared to indirect methods as there is no signal amplification provided by the use of secondary antibodies. Several secondary antibodies may bind to the primary antibody, resulting in an amplified signal. Background Non-specific binding is reduced. Samples with endogenous immunoglobulins may exhibit a high background.
Read the complete guide on direct vs indirect staining.2.2 Choosing a secondary antibody
Secondary antibodies are raised against a specific type of antibody from a certain species; this is the isotype of the primary antibody, e.g., rabbit IgG. Secondary antibodies are used to detect the primary antibody by binding to the primary antibody. They can bind the primary antibody in more than one place. Here are some things that you need to consider when choosing a secondary antibody for your experiment.
Host species
The secondary antibody binds specifically to the IgG of the host species used to generate the primary antibody. For example, if you use a primary antibody raised in rabbit, you will need an anti-rabbit secondary antibody raised in a host species other than rabbit (e.g., donkey anti-rabbit secondary).
The experimental procedure
Secondary antibodies come in conjugated forms. For applications such as ELISA or Western blotting enzyme-linked secondaries tend to be the most popular, whereas for flow cytometry or immunofluorescence, there is a preference for secondary antibodies conjugated to fluorescent proteins or dyes such as Alexa Fluor®.
Class/Subclass of antibody
The secondary antibody has to be directed against the isotype of the primary antibody. Polyclonal primary antibodies are typically raised in rabbit, goat, sheep or donkey and are generally IgG isotypes. Therefore the secondary antibody will usually be an anti-IgG H&L (Heavy & Light chains) antibody.
Monoclonal primary antibodies are commonly raised in mouse, rabbit and rat. For example, if the primary monoclonal antibody is a mouse IgG1, you will need an anti-mouse IgG or a less specific F(ab) fragment anti-mouse IgG.
Classes or isotypes: IgG (γ heavy chains), IgM (α), IgE, IgD (δ)
Subclasses: IgG1 (γ1 heavy chains), IgG2 (γ2), IgG3 (γ3), IgG4 (γ4), IgA1 (α1), IgA2
Types: κ light chain, λ light chain
Subtypes: λ1, λ2, λ3, λ4
Pre-adsorbed secondary antibodies
Pre-adsorbed secondary antibodies are ideal for eliminating species reactivity in multi-color experiments when several primary antibodies and their corresponding secondary antibodies are used simultaneously. Pre-adsorption (also referred to as cross-adsorption) is an extra purification step introduced to increase the specificity of an antibody. The pre-adsorption process reduces the risk of cross-reactivity between the secondary antibody and endogenous immunoglobulins present on cell and tissue samples.
F(ab) fragment antibodies
F(ab) and (Fab')2 fragment antibodies eliminate non-specific binding between Fc portions of antibodies and Fc receptors on cells (such as macrophages, dendritic cells, neutrophils, NK cells and B cells), and penetrate tissues more efficiently due to their smaller size.For more information on how to choose the right secondary antibody for your experiment, get the full guide.2.3 Conjugate selection
Secondary antibodies can be conjugated to enzymes, biotin, or fluorescent dyes/proteins. The choice of conjugate is dependent upon the experimental application and allows for colorimetric, fluorescent or chemiluminescent detection of primary antibodies in cell imaging, immunohistochemistry, flow cytometry, and western blotting applications.
You typically need a fluorescent conjugate for cell imaging, flow cytometry, and immunohistochemistry of frozen samples (IHC-Fr). Fluorescent-conjugated secondary antibodies allow for good signal amplification of your primary antibody, as mentioned above.
Learn the difference between different antibody conjugates and how to choose the most appropriate for your application.2.4 Choosing your fluorophore
Understanding the fluorophores in your experiment is particularly important for multicolor experiments. In this short video, you'll learn about the spectrum, emission, and absorption of fluorophores.
Download our fluorochrome chart, which includes
– Aligned emission and excitation fluorescence spectra for 30 of the most commonly used flow cytometry fluorochromes including tandem dyes
– Easy visualization of some of the most popular lasers and filters across the fluorescence spectra
– The fluorescence channel and relative brightness for each of the fluorochromes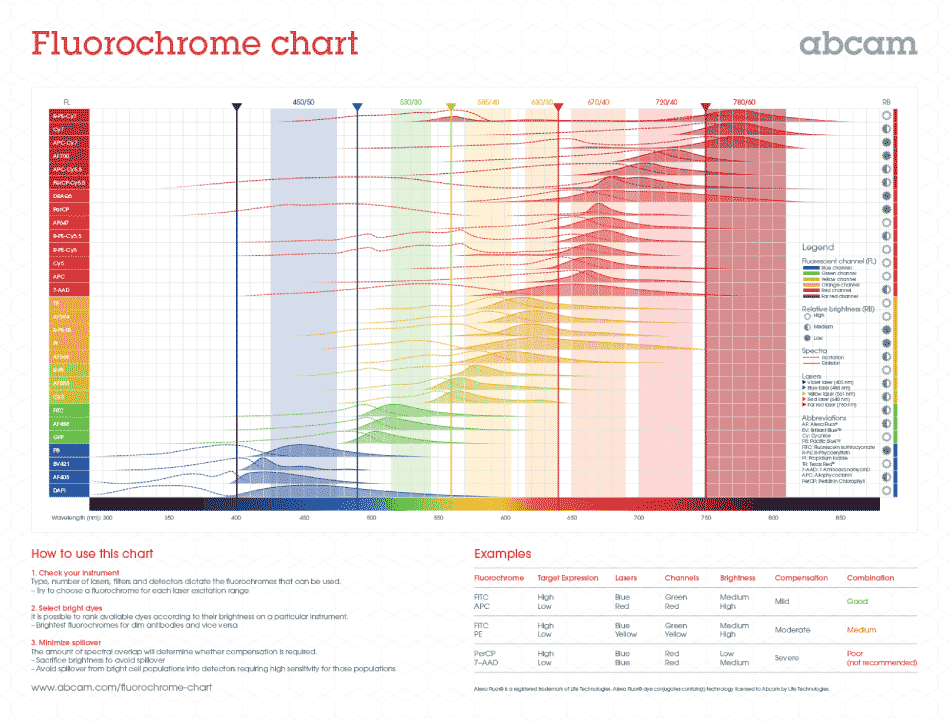
SummaryThat's the end of Part 2. You should now be better equipped to
– Decide between indirect and direct staining methods
– Select an appropriate secondary antibody
– Choose a suitable fluorophore or even multiple fluorophores for multiplex assays
In Part 3, we cover ICC and IHC protocols, sample preparation, immunostaining, and mounting, as well as giving an overview of the steps required for fluorescent staining and tips for staining more than one antigen.