-
Flow cytometry training
Part 1: know your flow cytometer
Welcome to our training series on flow cytometry. We begin with essentials like getting to know your cytometer, before taking you through some of the finer points and ending with how to get the most from this powerful technique.In Part 1 of flow cytometry training, we’ll take you through the core concepts behind the flow cytometer before moving on to fluorophores and how to select the right one for your needs.
Part 1 overview1.1 An introduction to flow cytometry
1.2 The flow cytometer
1.3 Understanding fluorophores
1.4 Choosing the right fluorophore
1.1 An introduction to flow cytometryFlow cytometry is a popular laser-based technology to analyze the characteristics of cells or particles. Flow cytometry is widely used to analyze the expression of cell surface and intracellular molecules, characterize and define different cell types in a heterogeneous cell population, assess the purity of isolated subpopulations, and analyze cell size and volume. It allows simultaneous multi-parameter analysis of single cells.
Two very popular applications for flow cytometry include:
• Immunophenotyping - identifying cell populations by the presence or absence of characteristic endogenous cell markers
• Fluorescence-activated cell sorting (FACS) of live cells - separating a cell population into subpopulations based on fluorescent labeling
In this video, we’ll introduce you to the various applications of flow cytometry and take you through the key elements of flow cytometric analysis such as forward and side scatter, gate strategy, and compensation.
1.2 The flow cytometerAs mentioned in the video above, a flow cytometer uses a combination of scattered light and emitted fluorescence to generate data. When running your sample (typically, a cell suspension) through the cytometer, sheath fluid hydrodynamically focuses the cell suspension through a small nozzle and past the laser in a single cell fashion (Figure 1). A detector then collects both forward and side scattered (FSC and SSC respectively) light, as well as fluorescence emitted from stained cells.
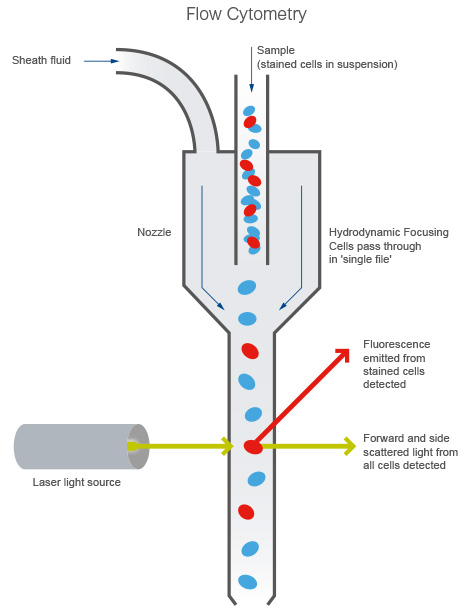
Figure 1. An overview of the flow cytometer.
FSC correlates with cell size, and SSC is proportional to the granularity of the cells, and this allows you to distinguish cell populations based on differences in their size and granularity alone.
Watch the video below to understand the importance of the different systems composing a flow cytometer and their different functions.
1.3 Understanding fluorophoresFluorophores (or fluorochromes) absorb and emit light within a range of wavelengths and are often conjugated to antibodies as detection reagents. The wavelengths at which a fluorochrome absorbs and emits light constitute what is known as the fluorochrome’s excitation and emission spectra respectively (or simply the fluorescence spectra).
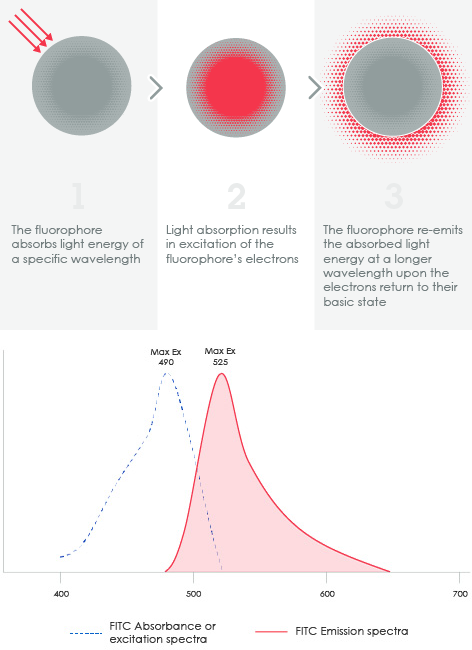
Figure 2. Top: excitation and emission fundamentals of fluorophores. 1) The fluorophore absorbs light energy of a specific wavelength. 2) Light absorption results in excitation of the fluorophore's electrons. 3) The fluorophore re-emits the absorbed light energy at a longer wavelength upon the electrons return to their basic state. Bottom: Excitation and emission spectra of FITC.
Learn more about fluorophores, including tandem dyes.
1.4 Choosing the right fluorophoreIt can be challenging at first to select the right fluorochrome for your experiments, especially when planning a multicolor experiment that uses two or more fluorochromes. We have developed a new selection tool to help you with this.
For multicolor experiments, you must be aware of the spectral overlap between fluorochromes so that you minimize problems like fluorescence spillover. Directly conjugated antibodies are best when doing multicolor experiments.
Below is a fluorochrome chart that allows you to quickly identify any potentially overlapping spectra from your fluorochromes.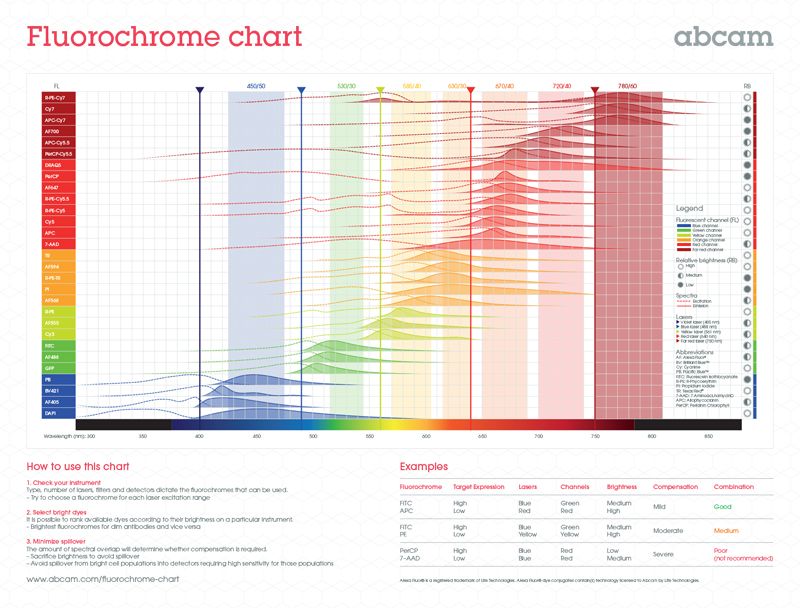
Download a full-size version of the fluorochrome chart.
With spectra covered, you need to think about the brightness of your fluorophores relative to the expression levels of your antigen. Some general rules:
• For low or unknown antigen expression or low cell numbers, you should use brighter fluorochromes, eg PE
• When you have an antigen with a high level of expression or high cell numbers, you can utilize dimmer fluorochromes, eg PerCP
Watch the short video below to learn more about choosing the right fluorophore.
SummaryThat’s the end of Part 1. You should now have a better understanding of:
• How the flow cytometer works
• Fluorophores and their varying spectra
• How to pick the right fluorophore for your experiment
In Part 2, we’ll cover sample preparation, appropriate controls, and staining protocols for flow cytometry.
Start Part 2 now!