Biophysical Quality Control: Our new standard for antibody validation
We’re constantly improving our antibody quality with recombinant technology, application testing and knock-out validation. Since 2010, we’ve withdrawn over 60,000 products that haven’t kept up with our rising standards.
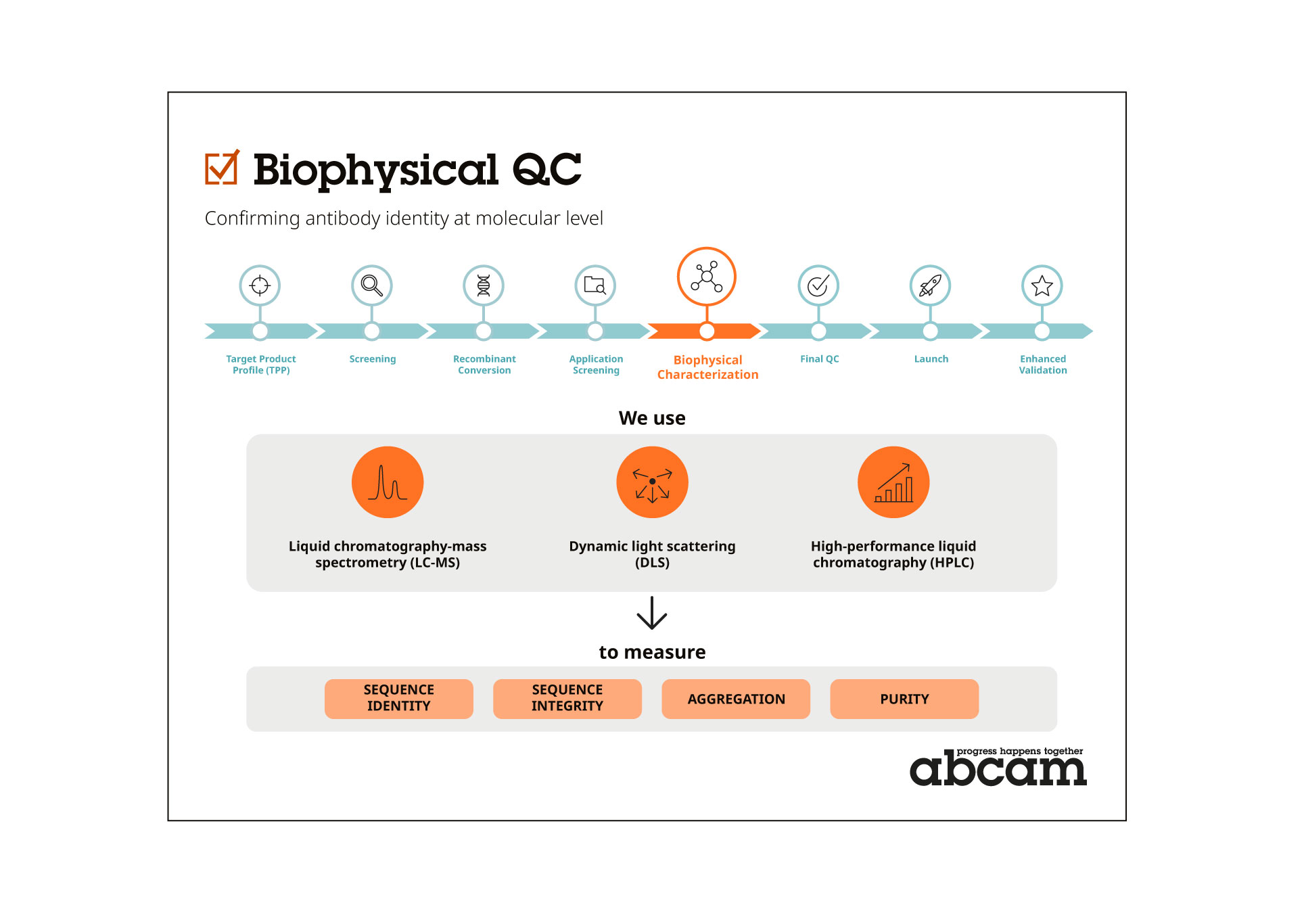
Now, we’re raising the bar even higher with an industry-leading initiative we call Biophysical Quality Control (QC): the biophysical characterization of our antibodies.
4 min read
Antibodies are a vital tool in life science research. But too often, the tool becomes a wrench in the works. Too many antibodies are poorly characterized, wasting critical time and resources on irreproducible research. A 2015 article in Nature identified that around 50% of spending on protein-binding reagents worldwide is wasted due to non-specific and inconsistent antibodies.
Overcoming the reproducibility crisis is at the heart of what we do at Abcam: providing antibodies with the highest possible specificity, sensitivity and consistency, so that researchers can keep moving forward with confidence.
That’s why we’re continually improving our antibody quality, using recombinant technology, extensive application testing, and knock-out validation to ensure specificity and high performance. We now offer more than 4,500 knock-out validated antibodies, with 400 added last year alone.
Now, we’ve taken the next step with a new industry standard, using Biophysical Quality Control (QC) to guarantee the best-performing antibody every time.
Biophysical QC detects any impurities and aggregates and provides us with a unique profile or ‘fingerprint’ of each product that’s not open to interpretation. We can use this data to validate subsequent batches, guaranteeing the highest specificity, sensitivity and consistency every time.
Eliminating lot-to-lot variability is important for long-term projects, enabling comparability of results across different stages. It also frees researchers from repeated re-optimization of their assay set-ups, giving them the confidence to pool batches from different production runs instead of a single lot, saving time and money.
Our dedicated Biophysical QC team has so far tested 75% of our portfolio of 32,000+ recombinant rabbit monoclonal antibodies.
We use a toolkit of tests established by the biopharma industry for therapeutic antibodies to assess sequence identity, sequence integrity, aggregation, purity, and concentration. These include liquid chromatography-mass spectrometry (LC-MS), dynamic light scattering (DLS) and high-performance liquid chromatography (HPLC). No other reagent supplier carries out this level of analysis, providing the highest level of assurance to researchers.
“When we introduce a validation technique, it can benefit thousands of researchers globally. It also helps set industry standards in antibody validation.”
Dr Alejandra Solache, Abcam’s Senior Vice President of Research and Development
“Our mission is to help scientists achieve their discoveries faster, and providing reagents that work the first time, every time, is key to fulfilling this goal,” says Dr Alejandra Solache, Abcam’s Senior Vice President of Research and Development.
Dr Solache has spent much of her career across industry and academia raising the quality and performance of research antibodies. In 2021, she won CiteAb’s Award for Significant Individual Impact for her leadership and dedication in addressing the reproducibility crisis.
“Abcam’s scale, influence and dedication to high quality put us in a unique leadership position with significant responsibility within the industry. When we introduce a validation technique, it can benefit thousands of researchers globally. It also helps set industry standards in antibody validation.”
Biophysical QC is the latest in our suite of validation techniques
The high quality of our antibodies is founded on a range of precise validation techniques. Biophysical testing builds on these tools to let you know our antibodies in detail, so you can have confidence in your results no matter what kind of assay set-up you’re using. They include:
Recombinant technology – for exceptional batch-to-batch consistency, sensitivity and specificity.
Extensive application testing – standard for all our antibodies, including immunohistochemistry (IHC) on tissue microarrays.
Advanced validation – antibodies validated in techniques specifically of interest for their target, including mass cytometry, ChIC/CUT&RUN and biological activity, identified by our ‘Advanced Validation’ tag.
Knock-out validation – performed using an extensive library of human knock-out cell lines, winning CiteAb awards in 2020 and 2022. Our in-house cell lines are also available for purchase.
Our innovation in biophysical quality control also won a Highly Commended Digital Science Tool of the Year award from CiteAb in 2024. Improving reproducibility can only bring a positive impact to research and health outcomes. By working together, we can tackle the reproducibility crisis and move science forward, faster.

 Back
Back